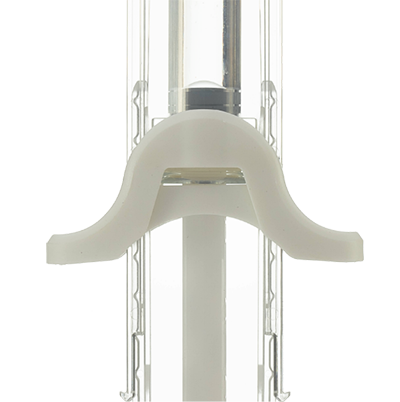
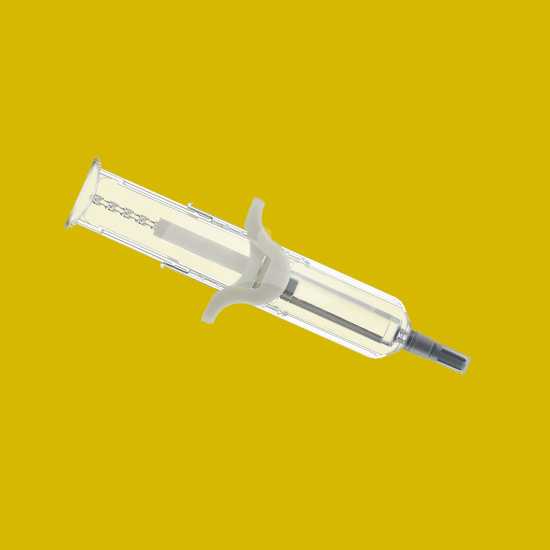
UniSafe® 2.25

View in
reality
A spring-free, passive safety device for 2.25mL pre-filled syringes, designed for simple assembly and use.
- Suitable for higher volume and higher viscosity formulations
- User confidence with an unobscured full drug visibility
- Simple final assembly
- Secure plunger prevents removal, spillage and repeat use
- Prevention of accidental activation
- Passive needle retraction and complete shielding
- Intuitive to use – same injection technique as UniSafe® 1mL
and a standard pre-filled syringe - Compatible with multiple syringe manufacturers
Specification
| Passive needle safety | Passive retracting needle | |
| Primary container/ syringe type | 2.25mL syringe standard cropped (cut) and small round flange | |
| Syringe size | 2.25mL staked 1/2” needle | |
| Fill volume | 0.2 – 2.25mL | |
| Needle insertion depth (subcutaneous) | 12.7mm (0.5inch) staked needle | |
| Needle shield type | Rigid needle shield | |
| Feasible injection angle | 45° – 90° | |
| Viscosity range – 0.5” 27G TW needle | Up to 100cP dependent on needle gauge* Tested at DV to 10cP with 27G TW needle and 2.25mL fill volume |
|
| Dose visibility | Full 2.25mL dose volume visible | |
| Disassembly/ Plunger removal force | >140N | |
| Weight – Subassembly & safety shroud | 11.3g | |
| Colours (standard finger flanges) | 6 including white |
*Dependant on temperature and injection time
Click on the +numbered icons to discover more about the device
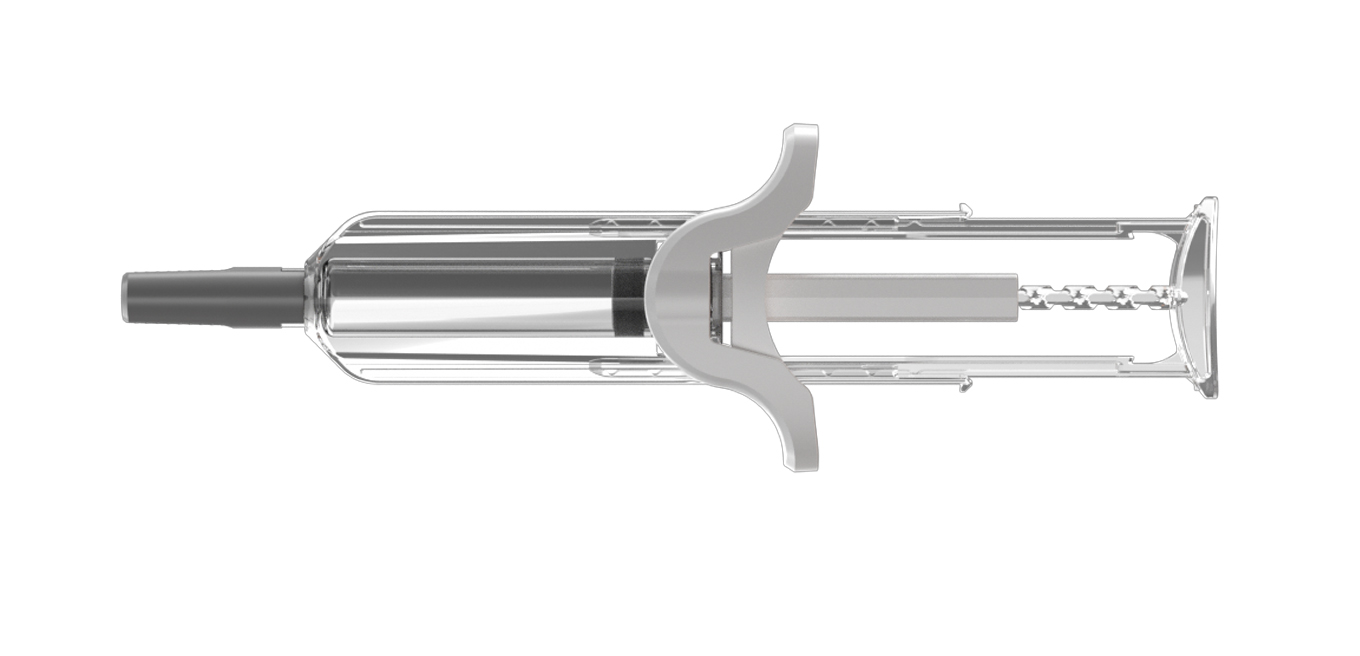
REDUCED RISK
Maintains sterility in assembly – plunger is fixed 2mm away from the stopper. The device design helps to address the potential risk of container closure integrity (CCI).
REASSURANCE
Large, ergonomic plunger head and a smooth, finger flange, resulting in an integrated look and feel.
SIMPLE FINAL ASSEMBLY
Final assembly process is simple and outside of the sterile filling area. The plunger rod does not screw into the stopper.
USER CONFIDENCE
Syringe barrel is unobscured, allowing the user to check the contents of the syringe, and to confirm the full dose has been delivered. UniSafe cannot be reused or prematurely activated.
PASSIVE SAFETY
Passive needle retraction means that the device is safe as soon as the plunger is fully depressed.
RELIABILITY
No risk of pre-activation in transit, manufacturing and removal from packaging.
COST EFFECTIVE
Designed to work with standard, 2.25 pre-filled syringes both cropped and small round flange
providing choice of primary container.
SECURE PLUNGER
Plunger rod cannot be pulled out when removing the RNS or the device from packaging, eliminating risk of accidental drug spillage and compromised sterility.